How lead acid batteries work
Let's be straight up right now; this is not an exhaustive exposé of the complexities of how a battery works, because believe me, it gets really complex and there are many good explanations out there. What we're trying to do here is give you an idea of the basic chemistry of lead acid batteries - and in the next page, lithium batteries - to give you an understanding of just why lithium batteries work so much better than lead acid batteries.
It may get a bit eye-rolling, but since you're here, I'm guessing you're somewhat interested. Let's go...
What's in a name?
There are many names for lead acid technology: AGM, lead acid, gel, flooded cell, lead crystal, deep cycle, start battery, hybrid, marine, doorstop - it’s all basically the same 19th century technology.
A complex chemical reaction
The diagram below is basically saying that a complex chemical reaction takes place.
In the process of discharging a lead acid battery, lead on the negative plate is dissolved by sulphuric acid to form lead ions, leaving fee electrons on the negative plate that can travel through an external circuit. These electrons are the electricity that powers your RV.
On the positive plate, the lead oxide of the plate is attacked by the positive hydrogen ions in the sulphuric acid, stripping the oxygen from the lead oxide to initially form OH ions and then combine with hydrogen ions to form water.
The lead ions from both the lead plate and the lead oxide plate combine with the sulphate from the sulphuric acid to become lead sulphate.
The opposite of all this movement of ions and electrons occurs during charging.
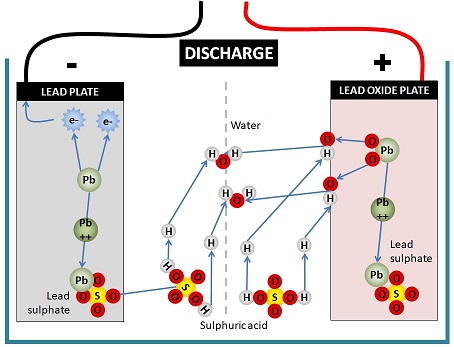
There are 3 products of this chemical reaction:

The faster you try to add or remove energy from the battery, the faster this chemical reaction has to be, and the more electrolysis and heat will be produced compared to useful power. This build-up of electrolysis and heat reduces the efficiency of the chemical reaction so that the faster you try to charge or discharge, the less of the total battery capacity you can have.
This is known as the Peukert Factor and there's not a thing you can do about it,If you take power out and put it back in slowly, you minimise these two unwanted reactions.
Every time you go through this discharge and recharge process, some of the lead plate is eaten away. When the lead’s gone, so is the capacity of the battery.
High internal resistance
The complexity of the process of a chemical reaction and the high energy consumption of that process means a lead acid battery has a high internal resistance.
In other words, the very process of creating power for you to use also wastes quite a lot of power. A low internal resistance means that very little energy is wasted in the process of creating power.
Lithium batteries have a very low internal resistance. To find out why, see How lithium batteries work.
Lead acid battery capacity
The accepted industry measurement of the capacity of a lead acid battery is known as the C20 rate. This is the capacity divided by 20 hours:
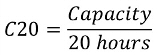
For example, a battery rated at 100Ah:
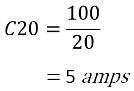
The C20 rate means that you can have 5 amps per hour for 20 hours until the battery is completely drained.
The commonly accepted safe discharge level for a lead acid battery is 50%. So now you can have 5 amps for 10 hours from a 100Ah battery.
If you try to remove the energy faster than the C20 rate, you will not get 10 hours before you have removed 50% of the capacity. This is known the Peukert Factor. The faster you try to take power from the battery, the less power you can have, because some of the electrical energy has been converted to heat energy and electrolysis splitting the water into hydrogen and oxygen.

A simple explanation of how lithium batteries work that explains their superior performance over lead acid batteries.
Many different chemistries come under the banner of 'lithium', but they all have different efficiencies and stability. It's important to know that not all lithium chemistries are suited to RV use.